Prostate cancer (PCa) remains a key challenge for men’s health and a significant economic burden on society. Majority of primary prostate tumors are treated successfully by radical prostatectomy or external beam radiotherapy, but tumors can progress to invasive and disseminated disease. Patients with metastatic PCa are treated with androgen deprivation therapy; whereas the initial response is very high, complete remissions are rare and disease progression resumes after a median of 2-3 years. The emergent castration-resistant PCa (CRPC) may respond to recently developed AR inhibitory drugs, but this is also short-lived as resistance develops in a few months after which prognosis is very unfavorable. The basis of androgen deprivation therapy is a large body of research implicating androgen signalling in all phases of PCa. The effects of androgens are mediated by the androgen receptor (AR), an androgen regulated transcription factor. AR signaling is critical to the development and function of the normal prostate, in the progression from primary to metastatic disease, as well as a hallmark of CRPC. The downstream pathways regulated by AR may also be important in the final stage of AR-independent disease. Therefore, discovering mechanisms of AR-driven gene regulation and their downstream effects in tumor cells is imperative. Despite extensive global analyses of AR target gene expression, their functional characterization and relevance in PCa is still limited at present. We have previously identified components of AR signalling and its crosstalk with other signalling pathways, including potential biomarkers and therapeutic targets. In particular, our efforts focus on a few genes cloned in our laboratory, such as the six transmembrane protein of prostate 1 and 2 (STAMP1 and STAMP2, also known as STEAPs), that are AR regulated and/or highly restricted to prostate for expression. We have shown that these proteins are essential for PCa growth and survival and that they impact a number of key cellular signaling pathways. We are currently characterizing these proteins functionally, their interacting partners, and how they steer PCa growth, as well as exploring methods to block their function for potential translational applications. We have also discovered that androgen signalling interfaces with endoplasmic reticulum stress pathways (summarized in the other project description); we now have a major effort in this area to functionally characterize this crosstalk and explore important nodes as potential biomarkers and therapeutic targets in model
The very unfavorable microenvironment of solid tumors with low pH, low oxygen tension, and deficient nutrient supply, as well as oncoprotein action, results in accumulation of misfolded proteins and metabolic disturbances that can signal cell death. Cancer cells have developed the capacity to survive these adverse conditions through precise modulation or ‘hijacking’ of stress pathways in the normal physiology. One central stress signaling pathway that is important in normal physiology and is also involved in disease states is endoplasmic reticulum (ER) stress. When there is undue stress on the normal cell, ER stress protective pathways, collectively termed the Unfolded Protein Response (UPR), are activated to maintain cellular homeostasis. When the chronic ER stress cannot be resolved, UPR instead activates cell death pathways. The cancer cell uses the cytoprotective aspects of the UPR for survival and skillfully steers the ‘double-edged sword’ of UPR. We recently showed that canonical UPR pathways are directly regulated by androgen receptor (AR) signaling in PCa cells and are critical for tumor survival and growth. We found that AR activated the inositol requiring enzyme 1 alpha (IRE1alpha) pathway by directly increasing IRE1alpha gene expression as well as those of IRE1alpha target X-Box Protein 1S (XBP-1S, a major UPR transcription factor) target genes. Consistently, there is strong concordance between AR expression and IRE1alpha pathway mRNA gene expression in multiple large cohorts of human PCa, including in CRPC. Furthermore, genetic targeting of IRE1alpha or XBP-1S, or small molecule (MKC8866) targeting of IRE1alpha, strongly inhibited PCa cell growth in vivo. We have shown that at least part of the mechanism of this is by direct activation of the oncogene c-MYC expression. MKC8866 is now in a Phase 1 Clinical Trial. We have also found that one of the other canonical UPR pathways, protein kinase R- like endoplasmic reticulum kinase (PERK) – Activating Transcription Factor 4 (ATF4) pathway, is regulated by AR signaling and has important roles in PCa growth in vitro and in vivo. We have identified a number of novel ATF4 target genes and have shown that they have important roles in PCa biology in vitro and in vivo. For example, we found that mitochondrial one carbon (m1C) cycle gene expression is activated by ATF4 in PCa cells that may serve as biomarkers or therapeutic targets. In another example, we have shown that the ATF4 target FAM129A serves as a feedback regulator of PERK-ATF4 signaling by divergent effects at different levels of the pathway. To identify the molecular framework for the UPR, we are carrying out genome wide knockout screens followed by molecular and cell biological characterization, as well as computational modeling. We have generated novel UPR reporter cell lines that are facilitating this work. We aim to help establish a comprehensive basic understanding of the UPR as well as exploring the possibility that its important nodes may serve as biomarkers or therapeutic targets in cancer, and perhaps in other diseases as well.
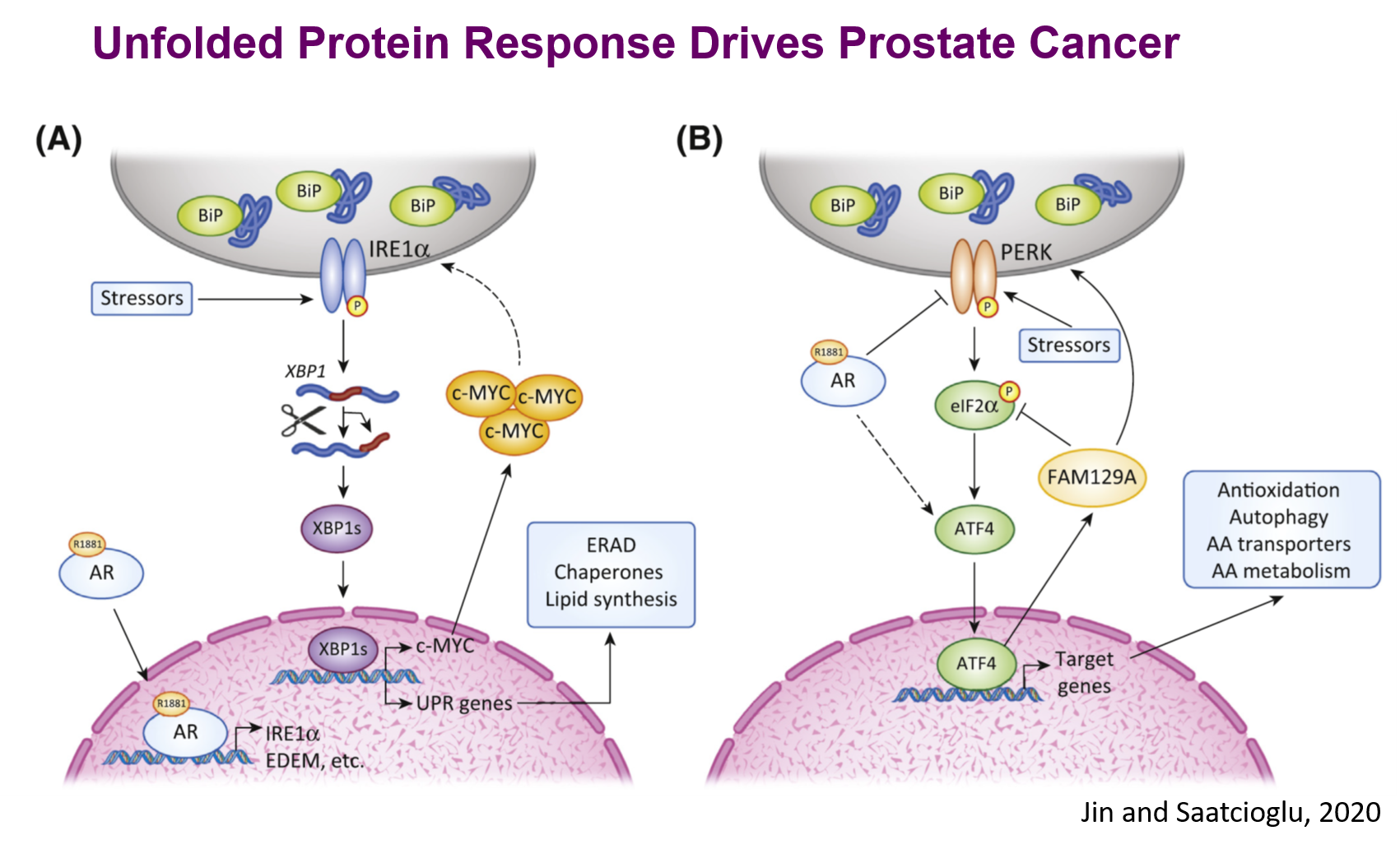
This work aims to contribute to basic understanding of hormone action and stress signaling, as well as identifying novel biomarkers and therapeutic targets for cancer. We are pursuing these goals through two related research foci: